New expanded indication will increase home hemodialysis device options available to patients with end-stage kidney disease
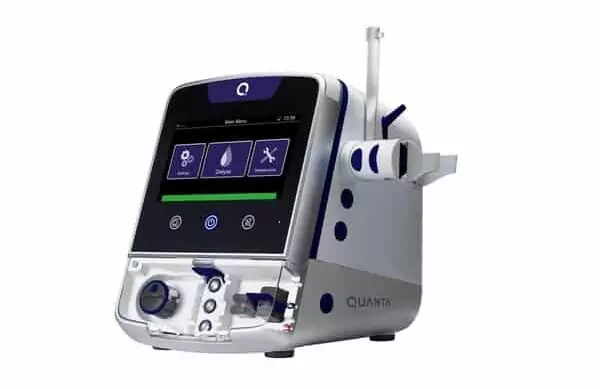
Seroba’s portfolio company, Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, today announced that it has submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for indication expansion of the Quanta™ Dialysis System, a compact and easy-to-use hemodialysis device. The Quanta Dialysis System is currently indicated for use in chronic and acute care settings. The new submission seeks to expand use to include self-care, in-home hemodialysis.
According to the U.S. Renal Data System, there are over 490,000 people in the U.S. who use hemodialysis to treat their end-stage kidney disease (ESKD). Home hemodialysis (HHD), which replaces the thrice-weekly commute to a dialysis clinic to receive treatment, accounts for 2% of the total hemodialysis population. (i) Studies suggest that HHD has many benefits over traditional in-center dialysis such as improvements to quality of life, health outcomes and access to care. In response to the benefits of HHD, the Centers for Medicare and Medicaid Services (CMS) established a program to encourage greater use of home dialysis and kidney transplants for Medicare beneficiaries.(ii)
Alejandro Galindo, Quanta Chief Executive Officer.The FDA submission for in-home use of the Quanta Dialysis System represents a significant milestone for Quanta in our journey to make dialysis more accessible to every patient in every setting. The Quanta Dialysis System is already providing patients in the U.K. with the freedom and flexibility that HHD offers. With clearance of this 510(k), patients in the U.S. will have more control over their care, and choice in where, when and how they receive treatment.”
This submission, pending regulatory clearance from the FDA, would make the Quanta Dialysis System the third device in the United States indicated for HHD in patients with end-stage kidney disease (ESKD). FDA clearance for the expanded indication is expected in 2024. Quanta’s 510(k) submission is supported by clinical data from its Home Run™ study, a prospective, multi-center, open-label trial to assess the efficacy and safety of the Quanta Dialysis System for HHD. Full results from the study will be shared this November during a poster presentation at the upcoming American Society of Nephrology (ASN) Kidney Week in Philadelphia, Pennsylvania.
About End-Stage Kidney Disease
End-stage kidney disease is the final stage of chronic kidney disease, where kidney function has declined enough to the point where the kidneys can no longer function on their own. With fully functioning kidneys, waste and excess fluids from the blood are filtered and then excreted in urine. For those with ESKD, the kidneys lose their filtering abilities and dangerous levels of fluid, electrolytes and waste build up in the body. Approximately 800,000 people in the United States are living with ESKD, 71% of which rely on dialysis to manage the disease, while the remaining 29% have a functioning kidney transplant.
About Quanta Dialysis Technologies
Quanta Dialysis Technologies is committed to making dialysis accessible to every patient in every setting with its Quanta Dialysis System. As a portable device with performance comparable to larger, traditional machines, the Quanta Dialysis System is a modular and powerful solution that provides the clinical versatility needed to deliver dialysis care across multiple settings. With a simple-to-use and intuitive user interface, it is designed to be operated by a broad range of users to bring dialysis directly to patients.
The Quanta Dialysis System is commercially available in the United Kingdom for home and hospital use. In the United States, it is FDA-cleared (K222067) for use in chronic and acute care settings, with treatment types including intermittent hemodialysis (IHD), sustained low efficiency dialysis (SLED), prolonged intermittent renal replacement therapy (PIRRT), slow continuous ultrafiltration (SCUF), and continuous venovenous hemodialysis (CVVHD).
(i) United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2022.
(ii) Centers for Medicare and Medicade Services. ESRD Treatment Choices (ETC) Model.
SRD Treatment Choices (ETC) Model
Further information about Quanta